In order to answer this question, we need to know that the following relation is constant:
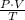
Where P is the pressure, V is the volume and T is the temperature.
So, for a constant temperature, the pressure and volume are inversely proportional.
Therefore, if the volume expands to double the initial volume, the pressure will decrease to half the initial pressure, so the relation above remains constant.
The amount of atoms in the region is still the same, so if the volume doubles, the density is halved.
So the correct option is A.