The reaction they give us is balanced. We must determine the moles of aluminum that are equivalent to that 45 g, for this, we will use the molar mass of aluminum equal to 26.98g/mol
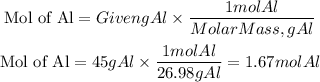
Now, in the reaction, we can see that for every 2 moles of aluminum, 1 mole of aluminum sulfate is produced. We can also use the molar mass of aluminum sulfate (101.96g/mol) to calculate the grams of aluminum sulfide produced.
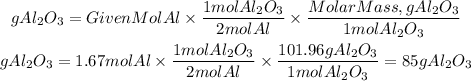
The grams of aluminum sulfate produced is 85g
The percent yield is calculated with the next equation:
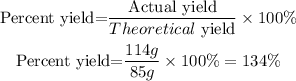
Percent yield=134%
This is not experimentally possible