Caitlin travels 4.5 miles at 11/3 hours.
So his rate of travel will be
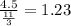
His rate is 1.23 mile/hour
Again Martin travels 4.25 miles at 11/5 hours
So his rate will be
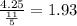
Hence martins rate is 1.93 miles/hour.
SO Martin travels at a greater rate than Caitlin.