Answer:
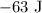
Step-by-step explanation:
Here, we want to know the amount of energy that was released as heat
Mathematically, we know that:
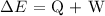
Where:
delta E is the change in Energy
Q is the heat
W is the work done
Substituting the values, we have it that:
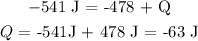
Kindly note that Q is negative because energy is released by the system
Also, W is negative as work is done by the system