The experiment involves the following reaction:
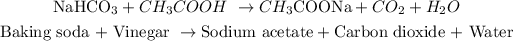
We have that 1 mole of Baking soda reacts with one mol of vinegar to produce one mole of carbon dioxide. Now, let's define what the actual yield is: Moles of the product that we have during the experiment.
Now, we have a relation between the moles and the volume. 1 mol CO2=24 L CO2. If we have the collected volume of CO2=340mL, the moles will be:
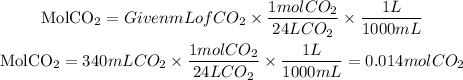
So, the actual yield of carbon dioxide is 0.014mol