Answer:
a) Towards the left
b) Towards the left
c) Towards the left
d) Towards the right
e) Towards the right
Explanations:
What is the Le Chatelier's principle?
This prnciple states that if a dynamic equilibrium is disturbed by changing the conditions, the position of equilibrium shifts to counteract the change to reestablish an equilibrium.
According to the equation shown below;
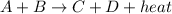
a) Removing some of the compound A means the total concentration of the reactant will reduce and be less than the product. In order to establish equilibrium, the equilibrium position will shift to the side with less amount of concentration of compound (in this case, the reactant side which is towards the left)
b) Increasing the temperature will decrease the equlibrium constant. This will favor the endothermic reaction (heat absorbed from the surroundings) and as such the equilibrium will shift to the left to absorb the added energy to the reactant.
c) Adding more compound D to the product will favour the backward reaction since the total concentration of the product will increase and be more than the reactant. In order to establish equilibrium, the equilibrium position will shift to the side with less amount of concentration of compound (in this case, the reactant side which is towards the left).
d) Increasing concentration of compound B (the reactant) will favor the forward reaction and as such the equilibrium position shifting to the right in order to establish equilibrium.
e) Decreasing the temperature will increase the equlibrium constant. This will favor the exothermic reaction (heat released to the surroundings) and the equilibrium will shift to the right