We need to find the mass of 1.6x10^20 molecules of carbon monoxide
First, we must find the number of mol in the 1.6x10^20 molecules
For this, we need to use the avogadro's number ( 6.02*10^23 )
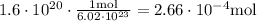
Then, we must calculate the molar mass of carbon monoxide
M(CO) = 12 g/mol + 16 g/mol = 28 g/mol
Finally, we must multiply the number of mol by the molar mass to find the mass
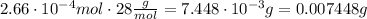
ANSWER:
0.007