ANSWER
The initial volume of the acid is 260mL
Step-by-step explanation
Given that;
The final volume of the ethanoic acid is 4.5L
The final concentration of the ethanoic acid is 1.0 mol/L
The initial concentration of the ethanoic acid is 17.4 mol/L
Follow the steps below to find the volume of the ethanoic acid
Step 1; Apply the dilution formula
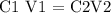
Step 2; Substitute the given data into the formula

Therefore, the initial volume of the acid is 260mL