To solve this problem we need to remember the equation for dilution of solutions:
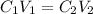
Where C1 is the concentration of the first solution, V1 is the volumen of the first solution, C2 is the concentration of second solution and V2 is the concentration of the second solution.
We know from the text that C1=0.15M and V2 is 150ml, and they tell us that V2 is obtained by adding V1 10ml which we can express as the following expression:
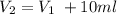
Solving for V1 and using the data provided:
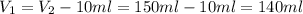
Now we have all de data for solving the first equation, we have to solve for C2 and substitute:
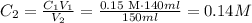
Final concentration is 0.14M