Step-by-step explanation:
We are given: mass of NH3 = 18.5 g
We know: molar mass of NH3 = 17.031 g/mol
: molar mass of H2 = 2.016 g/mol
We first determine the number of moles of NH3:
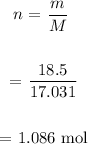
We then determine the number of moles of H2 from the number of moles of NH3:
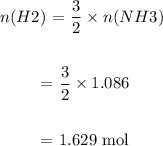
We then determine mass of H2 required:
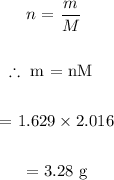
Answer:
grams of H2 required = 3.28 g