INFORMATION:
We have two acids:
- the first has a pH of 3
- the second has a pH of 6
And we must find how many times stronger is the acid with a pH of 3 than the acid with a pH of 6
STEP BY STEP EXPLANATION:
By definition,
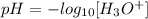
Now, we must find [H3O+] for the two acids
- First acid (pH = 3)
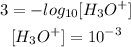
- Second acid (pH = 6)
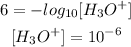
Finally, to find how many times stronger is the acid with a pH of 3 than the acid with a pH of 6 we must 10^-3 by 10^-6
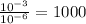
So, a pH of 3 is 1000 times as strong
ANSWER:
B. A ph of 3 is 1000 times as strong.