ANSWER
Step-by-step explanation
Given that;
The mass of oxygen is 0.54g
The mass of iron is 1.26g
Follow the steps below to find the percent composition of each element in the compound
Step 1; Define a compound
A compound is the combination of two of more elements that are chemically combined together
i.e., Iron + oxygen = Iron oxide
Step 2; Find the total mass of the compound
The total mass of the compound is calculated below as
Total mass = 0.54 + 1.26
Total mass = 1.8 grams
Step 3; Find the percent composition of each element by using the below formula
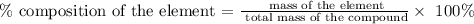
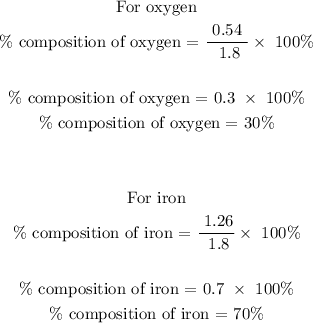
Therefore, the composition of Fe is 70% and the percent composition of O is 30