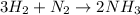
Reaction type: Combination (or Synthesis) reaction.
We are given:
mass of N2 = 61.802 cg = 0.61802g
mass of H2 = 61.802 cg = 0.61802g
Lets start by calculating the number of moles of N2 and H2.
For N2:
n = m/M where m is the mass and M is the molar mass
n = 0.61802g/28.0134
n = 0.0218 mol
For H2:
n = m/M
n = 0.61802g/2.01588
n = 0.07977 mol
So to know which one is the excess reactant, we have to divide the moles by thier respective co-efficient.
For N2: 0.0218 mol/1 = 0.0218
For H2: 0.07977 mol/3 = 0.0266
Therefore H2 is the excess reagent. Therefore the limiting reagent is N2 so we will use it to calculate the mass of NH3 produced, because the limiting reagent in a chemical reaction is the reactant that will be consumed completely. Once there is no more of that reactant, the reaction cannot proceed.
Masses after the reaction: We will use the stoichiometry to calculate these masses.
The molar ratio between N2 and NH3 is 1:2, therefore number of moles of NH3 is = 0.0218 x 2 = 0.0436 mol
mass of NH3 = n x M
m = 0.0436 mol x 17,031 g/mol
m = 0.743 g of NH3
Initially we had a mass of 0.61082g H2 and 0.61082 g N2 = 1.222g
mass produced is 0.743g which means 1.222 g-0.743g = 0.478g
Since H2 is the excess reactant, after the reaction there is 0.478g of H2
and all of N2 was used up since it is the limiting reagent. N2 = 0g after reaction.