Answer: 0.400L of argon contains 0.0179 moles of argon gas at STP
Step-by-step explanation:
The question requires us to calculate how many moles of argon gas are there in a 0.400 L, considering the standard temperature and pressure conditions (STP).
At STP, we can say that 1 mol of any gas corresponds to 22.4 L of this gas. Also, as a gas occupies the entire volume of the flask it is contained, we can assume that the volume of argon gas is 0.400 L.
Thus, we can calculate the number of moles of gas as:
22.4 L Ar ---------------------- 1 mol Ar
0.400 L Ar -------------------- x
Solving for x, we'll have:
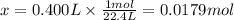
Therefore, 0.400L of argon contains 0.0179 moles of argon gas at STP.