For the third electron, the electronic configuration is,
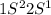
which shows that,
1. The value of the principle quantum number is,
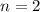
2. The value of the angular quantum number is,
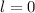
3. The value of magnetic angular quantum number is,
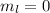
4. The value of magnetic spin quantum number is,
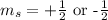
Thus, above four points represent the four quantum numbers of the third electron of multi-electron atom.