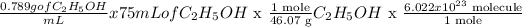We are given:
Volume of C2H5OH = 75 mL
density of C2H5OH = 0.789 g/mL
We know that 1 mole = 6.022x10^23 molecules
We can first get the number of moles of C2H5OH by calculating the mass from volume and density then convert mass to number of moles.
density = m/V
m = density x V
m = 0.789 g/mL x 75 mL
m = 59.175 g
Now we can find the number of moles. You can get molar mass of ethanol from adding the molar masses of 2C + 6H + O
n = m/M
n = 59.175 g/46,07 g/mol
n = 1.28 mol
Now that we know the number of moles, we can get the number of molecules.
1 mole = 6.022x10^23
1.28 moles = x molecules
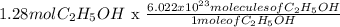
x = 7.735x10^23 molecules.
Therefore 75.0 mL C2H5OH has 7.735x10^23 molecules.
Summary of the calculation: