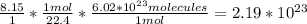Answer: 2.19x10^23 molecules
To find how many molecules there are, we have to find how moles of the gas there is. We know that 1 mole of any substance contains 22.4 Liters, so we divide the given amount by 22.4 to get to moles. Once you know the number of moles, you multiply it by 6.02x10^23 (Avogadro's number). Avogadro's number is how many atoms, molecules, or ions one mole of a substance contains. The complete equation would look like this: