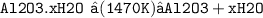Okay I will explain you friend...
To get pure alumina, you should follow these below steps.
Step (1):- Impurity alumina is treated with conc. NaOH solution to form sodium aluminate.
![{ \green{ \tt{Al2O3 + 2NaOH + 3H2O → 2Na[Al(OH)4]}}}](https://img.qammunity.org/2023/formulas/chemistry/college/vs3x7tk9jx2qgra2nzg831hktvpp4itzz5.png)
Step (2):- sodium aluminate is treated with carbon dioxide gas to form precipitated Al2O3
![{ \purple{ \tt{2Na[Al(OH)4] + CO2 → Al2O3.xH2O + NaHCO3}}}](https://img.qammunity.org/2023/formulas/chemistry/college/2o3euhnrmov6cgvfc6b3ta3elqphalnqw4.png)
Step (3):- Precipitated Al2O3 separated by filtration and heated at 1470K to give pure alumina.