ANSWER
Step-by-step explanation
Lithium chloride is produced as a result of direct combination of lithium and chlorine
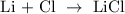
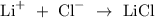
In the above reaction, lithium is a metal while chlorine is a non metal.
Recall, that most element participate in a chemical reaction so as to complete its octet structure.
Hence, LiCl is lithium chloride