Q.21:
We are asked what f(3) = 32 means?
In Q.20, we are given the following function
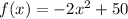
Where f(x) is the height (in feet) of the apple and x is the time in seconds.
If we substittute x = 3 seconds into the function, we get the corresponding height of the apple.
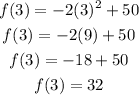
As you can see, the height of the apple is 32 feet at 3 seconds.
Therefore, the correct answer is the option (B)
The height of the apple at 3 seconds is 32 feet.