Answer:
6.5Joules
Explanations:
The formula for calculating the amount of heat that must be added is expressed as:
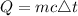
where:
• m is the ,mass ,of the object
,
• c is the s,pecific heat capacity
,
• △t is the ,change in temperature
Given the following parameters
m = 10grams
c = 0.13 J/g C
△t = 5 C
Substitute the given parameters into the formula
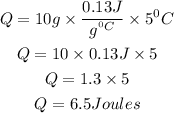
This shows that 6.5Joules of heat must be added to a 10 gram sample of Gold to increase its temperature by 5 C.