Answer:
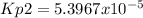
Step-by-step explanation:
Hello there!
In this case, since the Kp for the first reaction is given, according to the following equilibrium expression:
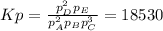
However, since the second reaction stands for the reverse of the initial one, the equilibrium expression would be:
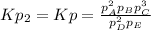
And therefore its Kp the inverse of the aforementioned one:
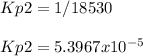
Best regards!