To prepare 1.0x10^-2M NaOH from 0.1M NaOH stock solution, you have to take a volume of the stock solution and dilute it using 9 times that volume of water. We conclude it from the equation for dilutions:
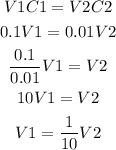
It means that to prepare the 1.0x10^-2 solution, dilute 1 mL (for instance) of stock solution in 9mL of water.