The question provides information on three trials where the volume of NaOH used in each one is provided and requests us to answer questions about the reaction between the 0.5140 M NaOH solution and an HCl solution with unknown concentration (volume of HCl = 10.0 mL).
The reaction between NaOH and HCl, a strong base and a strong acid, can be expressed as:
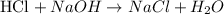
To calculate the number of moles of NaOH, we'll need the following equation, where the molarity of a solution (M) is defined as the number of moles of the compound (n) divided by the volume of the solution (V):
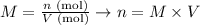
Since the average volume of NaOH used was 10.43 mL and the concentration given for this solution was 0.5140 M, we can calculate the number of moles of NaOH as it follows:
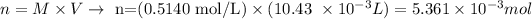
(notice that we must convert the volume, given in mL, to L, by multiplying the value by 0.001)
Therefore, the number of moles of NaOH calculated from the average volume used is 0.005361 moles.
To calculate the number of moles present in 10.0 mL of the unknown HCl sample, we'll consider that the average volume of NaOH used was the volume required to completely neutralize 10.0 mL of HCl. With that, we can write that, when the neutralization happens:
number of moles of NaOH = number of moles of HCl
Therefore, the number of moles of HCl in 10.0 mL of the solution is 0.005361 moles.
At last, we can calculate the concentration of the HCl solution using the number of moles calculated (0.005361 moles) and the volume used (10.0 mL), as it follows:
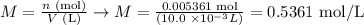
Therefore, the molar concentration of the HCl solution used is 0.5361 mol/L, calculated from the average volume of NaOH used in the experiment.