Step 1 - Understanding Avogadro's number (mole)
By definition, one mole is a number that corresponds to 6.02*10^23 unities of something. This number is called Avogadro's number.
Let's say, for example, we have one mole of chairs. How many chairs would we have? Exactly 6.02*10^23 chairs.
Step 2 - Using this information to solve the exercise
To solve the exercise, we can set the following proportion:
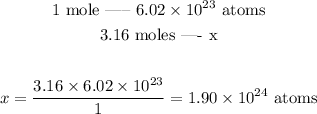
The total number of atoms we would have would be thus 1.90*10^24 atoms.