Given the function:
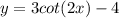
Let's find the vertical asymptotes of the function.
To find the vertical asymptote, set 2x equal to zero:
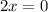
Solve for x.
To solve, divide both sides by 2:
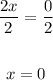
Also, term inside the cotangent function, (2x), to π:
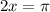
Solve for x by dividing both sides by 2:
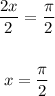
Also, apply the trigonometric identity:
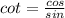
Hence, the vertical asymptote will be where sin(2x) equals 0.
Now, input all given choices for x in sin(2x) and solve:
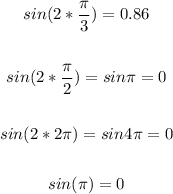
Therefore, the vertical asymptotes of the given function are:
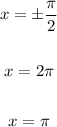
ANSWER:
B. x = ±π/2
C. x = 2π
D. x = π