There are 36.03 grams in 3.00 moles of carbon.
1st) We need to look for the atomic mass of Carbon (C) in the Periodic Table of Elements:
Carbon atomic mass = 12.01 g/mol
2nd) With the atomic mass of carbon and a mathematical Rule of Three we can calculate the grams of carbon in 3.00 moles:
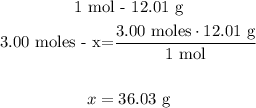
So, there are 36.03 grams of carbon in 3.00 moles.