ANSWER
The volume will be one-half of the original volume
option A
Step-by-step explanation
Given that;
The initial moles of the gas is 2 moles
The final mole of the gas is 1 mole
The mathematical representation of the Avogadro's law is given below as
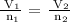
Let n1 = 2 moles and n2 = 1 mole
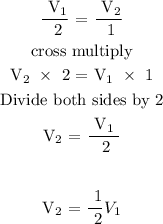
Therefore, the volume will be one-half of the original volume
option A