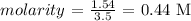Answer:
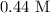
Step-by-step explanation:
Here, we want to get the molarity of the solution
Mathematically, we have that calculated as:
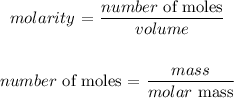
We start by getting the number of moles
We can get that by dividing the mass by the molar mass
The molar mass of NaCl is 58.44 g/mol
The number of moles is thus:
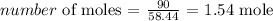
To get the molarity, we have it that: