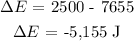Answer:
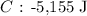
Step-by-step explanation:
Here, we want to get the change in the internal energy of the system
Mathematically, according to the 1st law of thermodynamics, we have it that:
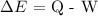
where:
Q = 2,500 J
W = +7,655 J
W is positive since work is done by the system
Substituting the values into the equation above, we have it that: