The chemical equation:
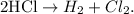
And the concentrations of hydrogen chloride:
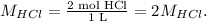
We do an ICE chart:
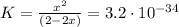
Doing the calculations in a software, we obtain that x is:
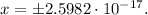
Using the positive result, we obtain that:
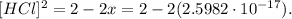
![\lbrack HCl\rbrack=\sqrt[]{2}\approx1.41\text{ M}](https://img.qammunity.org/2023/formulas/chemistry/college/gp0c4b6nnvtqfhenqsrt6mbpf7j7zeum97.png)
These results is tellinig us that the direction of the equilibrium in the reaction goes like this:
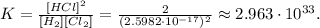
When K > 0, the direction goes to the right: to the products.