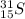The same chemical element can be constituted by different atoms, that is to say, their atomic numbers are equal, but the number of neutrons is different. These atoms are called isotopes. An isotope is represented as follows:
On the upper left side is written the mass number, i.e. the sum of protons and neutrons. And in the lower-left side is written the atomic number which refers to the number of protons.
The atomic number can be found in the periodic table, for sulfur (S), the atomic number is 16.
The mass number will be: Neutrons + Protons = 15+16 = 31
So, the sulfur (S) isotope with 15 neutrons will be: 31S