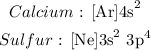Answer:
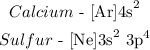
Step-by-step explanation:
Here, we want to write the noble gas configuration for the given element'
To do this, we consider the noble gas before them
The noble gas before calcium is Argon while the one before sulfur is Neon
We have the noble gas configuration as follows: