Consider that the area of a triangle with base (b) and height (h) is given by,
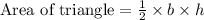
According to the given problem,
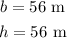
So the area of the triangle becomes,
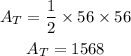
Consider that the area of the circle with diameter (d) is given by,
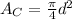
According to the given problem,
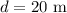
Then the area of the circle becomes,
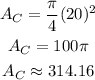
Now, the area of the shaded region (A) is calculated as,
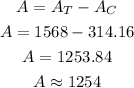
Thus, the area of the shaded region is 1254 sq. meters approximately.
Therefore, the 3rd option is correct choice.