In this case, we have NaOH as the limiting agent. Therefore the reaction will be according to the number of moles of NaOH present. We have the following amount of NaOH:
The molecular weight (MW) of NaOH: 40.0 g/mol
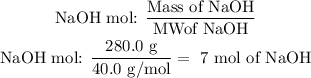
We have 7 mol of NaOH, so we will have 3.5 mol of Cu(OH)2. Because the ratio NaOH: Cu(OH)2 is 2:1. Now, we have to determine the grams of Cu(OH)2 using the molecular weight:
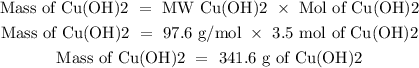
So, we will have 341.6 g of Cu(OH)2 precipitated after the reaction