First, find the molar mass of the compound NaCl. The molar mass of Na is 22.99 grams per mole, and the molar mass of Cl is 35.45 grams per mole. Then, add these molar masses 22.99+35.45 = 58.44 grams per mol.
Then, we use the ratio of 58.44 grams / 1 mol to find the number of moles in 25.0 g of NaCl.
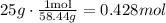
Therefore, there are 0.428 moles of NaCl.