We need to calculate the density of the bar of gold, using the following formula:
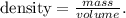
The units of density must be in kg/m^3 or g/cm^3. We can do the conversion from kg to g. Remember that 1 kg equals 1000 g:
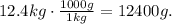
Now, we can apply the formula using the last data:
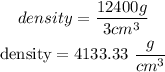
Let's see how would be the density in kg/m^3, remembering that 1 m^3 equal 1000000 cm^3:
![undefined]()