ANSWER
The % mass of the solution is 25%
Step-by-step explanation
Given information
The mass of solute = 25 grams
The mass of solvent = 75 grams
To find the % mass of the solution, follow the steps below
Step 1: Find the mass of the solution
Recall, that solution is the summation of the solute and its solvent
Solution = solute + solvent
Hence, the mass of the solution is calculated below as
Mass of solution = mass of solute + mass of solvent
Mass of solution = 25 + 75
Mass of solution = 100 grams
Step 2: Find the % mass of the solution by using the formula below
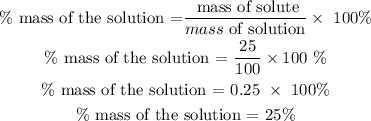
Hence, the % mass of the solution is 25%
Option B