The reaction is balanced, so we can proceed with the calculations. To solve the question we will follow the following steps:
1. We calculate the moles of C8H18, dividing the mass of the fuel by its molar mass. The molar mass of C8H18 is: 114.23g/mol.
2. By stoichiometry we find the moles of CO2 that are produced from the moles of C8H18 found.
3. We find the volume of CO2 formed using the ideal gas law which tells us:

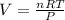
Where,
P is the pressure of the gas in atm, 1.00 atm
T is the temperature of the gas in Kelvin, 21.0°C=294.15K
R is a constant, 0.08206atm.L/mol.K
n is the number of moles of the gas
V is the volume of the gas in liters
Let's continue with the calculations.
1. Moles of C8H18
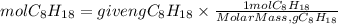
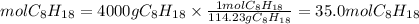
2. Moles of CO2
The ratio CO2 to C8H18 is 16/2, so the moles of CO2 formed will be:
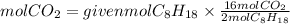
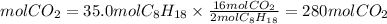
3. Volume of CO2
We replace the known data in the gas ideal law:
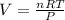
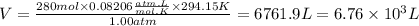
Answer: Would be produced 6.76x10^3 liters