ANSWER
Step-by-step explanation
Given that;
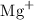
The atomic number of magnesium is 12.
In a neutal atom, the atomic number is equal to the number of proton
Therefore, the number of proton is 12
The magnesium ion lose one electron and it has 11 electrons
Hence, the Bohr model of Mg+ is drawn below as