In order to find if the points lie on the same line, let's find the line that passes through the first two points, then we check if the third point is on the line.
We can apply the points in the slope-intercept form of the line:
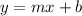
Applying the points, we have:
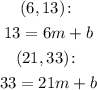
Subtracting the second and the first equation, we have:
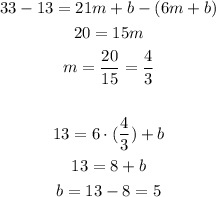
So the equation of the line is y = 4/3x + 5.
Testing the third point, we have:
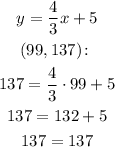
The result is true, so the point is on the line.
So both sentences are True.