Step 1 - Understanding how pressure and volume are related
There are three main variables that can modify the state of a gas: pressure, volume and temperature.
When one of these variables is kept constant, the remaining two become proportional to each other. Suppose temperature is kept constant. In this case, pressure and volume become proportional.
I.e., if we increase the pressure, we also increase the volume. We can state it mathematically as follows:
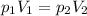
We can use this equation to solve this exercise.
Step 2 - Calculating the new volume
According to the exercise:
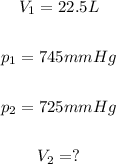
Setting these values in the equation given in step 1:
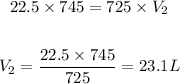
Answer: 23.1L