Step-by-step explanation:
The expression is given below as
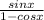
Hence,from the expression above,

Hence,
The first step is for us to rationalize the denominator by multiplying both the numerator and denominator by its conjugate
The conjugate is given below as
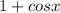
By rationalizing the denominator, we will have
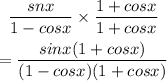
Hence,
The final answer is
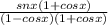
OPTION D is the correct answer