Answer:
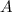
Step-by-step explanation:
Here, we want to select which of the options represent the property of an acid
An acid like hydrochloric acid reacts with metallic elements (for example, group 2 elements) like magnesium to produce hydrogen gas and the corresponding metallic salt
We can see that as follows:
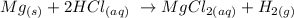
Thus,the correct option here is that A mineral acid react with some metals to produce hydrogen gas