Q. 4:
Recall Newton's second law of motion,
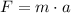
Where F is the force, m is the mass, and a is the acceleration of the object.
Re-writing the above equation
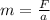
As you can see, if force is held constant then the mass of the object is inversely proportional to the acceleration of the object.
Quadrupling the mass means 4 times the mass
![undefined]()