Answer
4Al + 3O2 ------> 2Al₂O₃
4 moles of Al₂O₃ will be produced
Step-by-step explanation
Given:
Moles of A = 8 moles
The given unbalanced equation is: __Al + __O2 --> __Al2O3
Solution:
The balanced equation for the reaction is:
4Al + 3O2 ------> 2Al₂O₃
From the balanced equation, 4 moles of Al produced 2 moles Al₂O₃
Therefore, 8 moles of Al will produce
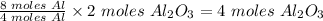
4 moles of Al₂O₃ will be produced.