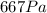According to Boyle's Law, an ideal gas at a constant temperature will satisfy the following relation between the initial and final conditions of pressure and volume:
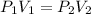
Isolate P_2 from the equation:
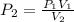
Substitute the values for the initial pressure as well as for the two volumes to find the value of the final pressure of the gas:
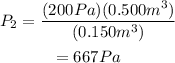
Therefore, the final pressure of the gas is: