Answer:
223.5K
Explanations:
According to general gas equation
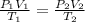
Given the following parameters
P1 = 1.0 atm (standard pressure)
V1 = 750mL
P2 = 2.0atm
V2 = 500mL
T2 = 25 + 273 = 298K
Substitute the given parameters
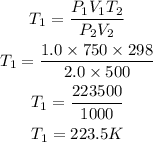
Hence the required initial temperature is 223.5K