Given the reaction:
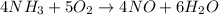
As you can notice, the reaction is actually balanced. So, we could use the coefficients of the reaction to find the volume of oxygen that reacts with 1.12 L of NH3.
First of all, remember that the Molar Volume statement tells us that at standard temperature and pressure, 1 mole of any gas occupies 22.4 L.
Then,
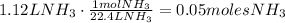
This is the amount of NH3 that we actually have. Now, according to the coefficients of the reaction, we can notice that 5 moles of Oxygen reacts for each 4 moles of NH3. So,
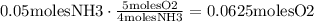
Now, using again the fact that 1 mole of any gas occupies 22.4 L, we obtain:
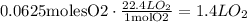
Therefore, the answer is 1.4L.