Answer
Molarity of the solution = 2.81 mol/L
Step-by-step explanation
Given:
Mass of lithium carbonate = 38 grams
Volume of solution = 183 mL
What to find:
Molarity of the solution.
Step-by-step solution:
The molarity of the solution can be calculated using the molarity formula, which is;
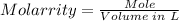
First, you need to convert 38 grams of lithium carbonate to mole using the mole formula.
Molar mass of lithium carbonate = 73.891 g/mol
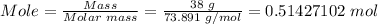
Also, Volume in L = (183/1000) = 0.183 L
Putting the values of mole and volume in L into the molarity formula above, we have;
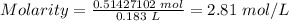
Hence, the molarity of the solution is 2.81 mol/L.